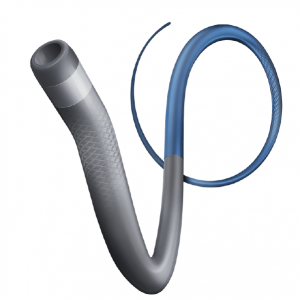
APERIO® Hybrid Thrombectomy Device
Manufacturer: ACANDIS
Category:Treatment of ischaemic strokes
- Effective hybrid cell design
- Outstanding visibility
- Treatment of vessel diameters from 1.5 – 5.5 mm
- Compatible with 0.021’’ – 0.027’’ ID microcatehters
Description
APERIO® Hybrid Thrombectomy Device
Clearly Visible
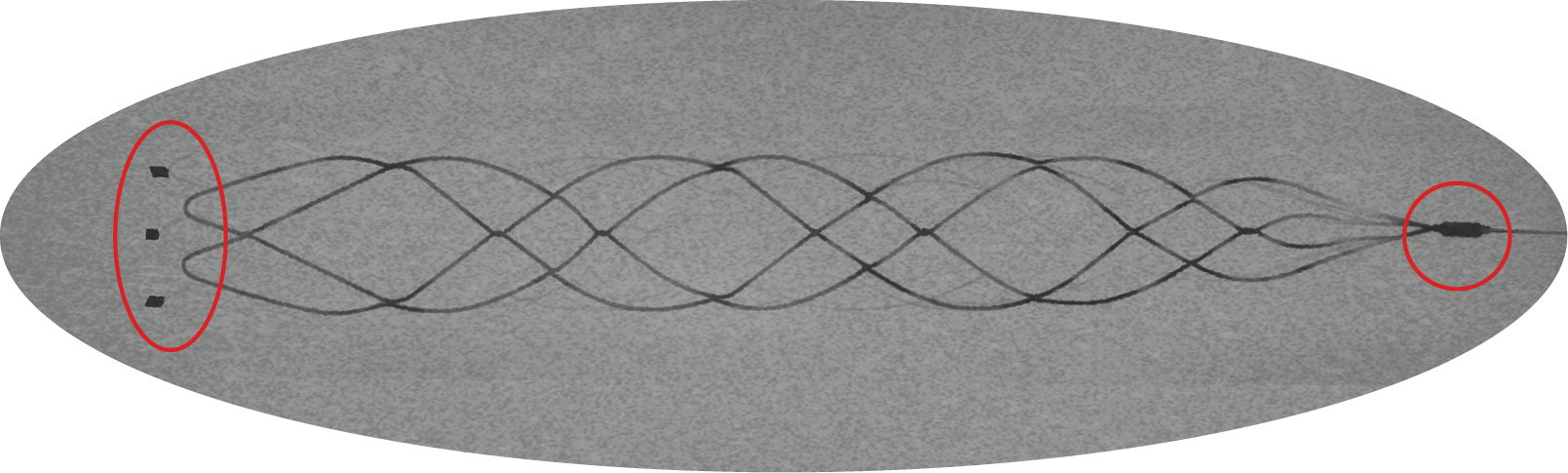
Clinical Experience
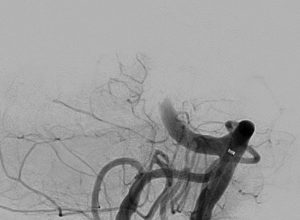
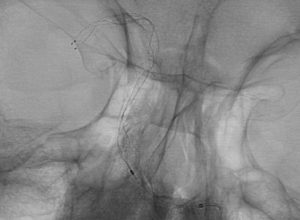
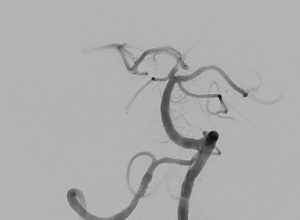
Images are courtesy of Dr. Christoph Kabbasch, University Hospital Cologne, Germany
Disclaimer:
Please consult the Instructions for Use for all indications, contraindications, warnings, cautions as well as possible adverse effects. Acandis® products are to be used exclusively by trained medical professionals. Orders are taken only in regions where the product is approved. Please contact an Acandis® representative for product availability.
Specification
| Labelled APERIO Hybrid Dimension (mm) | Reference Number | Device Diameter (mm) | Devicee Length (mm) | Recommended Vessel Diameter (mm) | Required Microcatheters for Delivery (inch) |
|---|---|---|---|---|---|
| 3.5 x 28 | 01-000704 | 3.5 | 28 | 1.5 – 3.0 | 0.021 |
| 4.5 x 30 | 01-000705 | 4.5 | 30 | 2.0 – 4.0 | 0.021 |
| 4.5 x 40 | 01-000706 | 4.5 | 40 | 2.0 – 4.0 | 0.021 |
| 4.5 x 50 | 01-000707 | 4.5 | 50 | 2.0 – 4.0 | 0.021 |
| 6.0 x 40 | 01-000708 | 6.0 | 40 | 3.5 – 5.5 | 0.021 – 0.027 |
| 6.0 x 50 | 01-000709 | 6.0 | 50 | 3.5 – 5.5 | 0.021 – 0.027 |